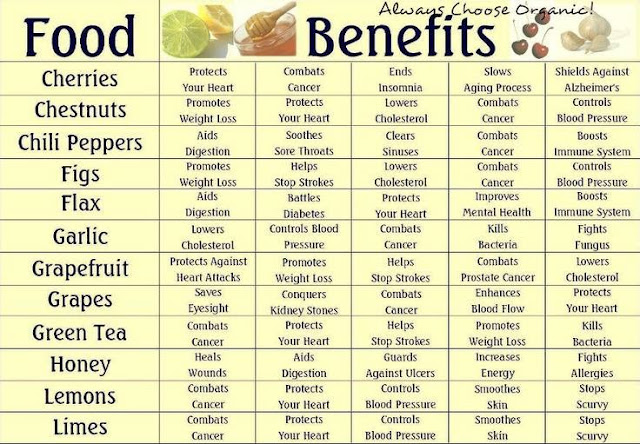
Everyday Living® Your information source for products, shopping, marketing and business services, health matters, home and garden, food and lesiure, weight loss, anti-ageing surgical procedures and non-surgical rejuvenation treatments. www.everydayliving.com®
Wednesday, November 29, 2017
Thursday, November 23, 2017
Tuesday, November 21, 2017
On the 20th anniversary of Canada's tainted blood inquiry, documents reveal the federal government worked with private pharmaceutical industry to undermine Krever's landmark recommendations
PRESS RELEASE
On the 20th anniversary of Canada's tainted blood
inquiry, documents reveal the federal government worked with private
pharmaceutical industry to undermine Krever's landmark recommendations
|
|
Tuesday November 21, 2017 (OTTAWA) - On
the eve of the 20th anniversary of the Krever Inquiry into the tainted
blood scandal, parliamentarians, health advocates and health
professionals gathered in Ottawa to call on the Trudeau government to
uphold the landmark Krever Inquiry and rescind licenses granted to
private, for-profit blood collection clinics that are selling Canadian
blood abroad.
Documents
obtained through a Freedom of Information request* implicate Health
Canada in working with the private blood broker's unregistered lobbyist
for years in the lead up to obtaining operating licenses, despite
pan-Canadian opposition. The controversial private clinics were banned
from operating in Ontario and Alberta to protect the blood supply.
"These
documents demonstrate a wanton disregard for the security of the
Canadian blood supply. It is alarming that a lobbyist and the private
pharmaceutical industry would be given an all access pass to make policy
decisions on behalf of the Canadian public," said Kat Lanteigne,
Executive Director and CoFounder of BloodWatch. "Prime
Minister Trudeau must step in and direct Health Canada to rescind
licenses granted to Canadian Plasma Resources immediately."
Canadian
Blood Services (CBS), the public agency created in the wake of the
Krever Inquiry, reported a decline in voluntary donors where the first
private clinic opened in Saskatoon. CBS has issued multiple warnings to
Health Canada and provincial governments to end support for the private
collectors, citing a major risk to the security of Canada's blood
supply.
"In New Brunswick, we've witnessed first-hand how private blood brokers threaten our fragile blood system," said Paula Doucet, President of the New Brunswick Nurses Union. "Without
any public consultation, Health Canada licensed a for-profit clinic in
Moncton that is located close to the University, competing directly with
our public system for the next generation of blood donors."
There are plans to open a third private clinic in Saint John, N.B., with more expansion plans for Manitoba, Nova Scotia and B.C.
"The
introduction of private, for-profit plasma collection sites is
threatening CBS' ability to collect enough plasma to meet Canadian
need," said Adrienne Silnicki, National Director, Policy and Advocacy,
Canadian Health Coalition. "The federal Health Minister must support our
public blood collector and reject those trying to profit off the blood
of Canadians."
BloodWatch
is calling for an official apology from the Prime Minister to those who
received tainted blood, federal recognition of November 26 as the day
the Krever Report was tabled in the House of Commons and immediate
action to rescind the licenses of all for-profit blood clinics.
An estimated 8,000 lives have been lost due to Canada's tainted blood tragedy.
-30-
|
|
For further information please contact:
Adrienne Silnicki, National Director, Policy and Advocacy: (613) 402-6793, asilnicki@healthcoalition.ca
Lauren Snowball,Communications Officer, CFNU: (613) 868-5702
Kat Lanteigne, Executive Director and CoFounder, BloodWatch: (647) 272-7381
Paula Doucet, President, New Brunswick Nurses Union: (506) 543-2255
*For access to the documents obtained through the Freedom of Information request, please email info@bloodwatch.org
|
|
The
Canadian Health Coalition is a public advocacy organization dedicated to
the protection and improvement of Medicare. You can learn more about
our work at healthcoalition.ca.
______________________________________________________________
|
|
COMMUNIQUÉ DE PRESSE
Alors que l'Enquête sur le scandale du sang contaminé au
Canada fête ses 20 ans, des documents révèlent que le gouvernement
fédéral a collaboré avec l'industrie pharmaceutique privée et est allé à
l'encontre des recommandations charnières du rapport Krever
|
|
Mardi, 21 novembre 2017 (OTTAWA) -
À la veille du 20e anniversaire de l'Enquête Krever sur le scandale du
sang contaminé, des parlementaires, des défenseurs des soins de santé et
des professionnels de la santé se sont rendus à Ottawa pour demander au
gouvernement Trudeau de respecter les recommandations de l'Enquête
Krever, et de retirer les permis accordés aux cliniques privées à but
lucratif de collecte de sang, qui vendent le sang canadien à l'étranger.
Des
documents obtenus dans le cadre d'une demande d'accès à l'information*
indiquent que, depuis des années, Santé Canada a collaboré avec des
lobbyistes non enregistrés du secteur des cliniques privées de collecte
de sang avant de leur accorder des permis, et cela malgré l'opposition
manifestée dans tout le pays. Ces cliniques privées controversées ont
été interdites en Ontario et en Alberta afin de protéger nos réserves en
sang.
« Ces
documents témoignent d'une insouciance totale envers la sécurité des
réserves de sang du Canada. C'est alarmant de constater qu'on puisse
donner à des lobbyistes et à l'industrie pharmaceutique privée le
pouvoir de prendre des décisions stratégiques au nom de la population
canadienne », souligne Kat Lanteigne, directrice générale et
co-fondatrice de BloodWatch. « Le premier ministre Trudeau doit
intervenir et ordonner à Santé Canada de retirer immédiatement les
permis accordés à Canadian Plasma Resources. »
La
Société canadienne du sang (SCS), agence publique créée dans la foulée
de l'Enquête Krever, fait état d'un déclin du nombre de donneurs
volontaires à Saskatoon où la première clinique privée a ouvert ses
portes. Plusieurs fois, la SCS a mis en garde Santé Canada et les
gouvernements provinciaux, et leur a demandé de cesser leur appui aux
cliniques privées de collecte du sang, précisant le risque important à
la sécurité des réserves de sang du Canada.
« Au
Nouveau-Brunswick, nous sommes directement témoins de la menace,
engendrée par les compagnies privées de collecte de sang, à notre
système fragile de collecte de sang », souligne Paula Doucet, présidente
du Syndicat des infirmières et infirmiers du Nouveau-Brunswick. « Sans
consultations publiques, Santé Canada a accordé un permis à une
compagnie privée qui a ouvert ses portes à Moncton, près de
l'Université, et qui est en concurrence directe avec notre système
public pour recruter la prochaine génération de donneurs de sang. »
On
prévoit ouvrir une troisième clinique privée à Saint-Jean (N.-B.),
ainsi que d'autres au Manitoba, en Nouvelle-Écosse et en
Colombie-Britannique.
« L'ouverture
de cliniques privées à but lucratif rémunérant les donneurs de plasma
menace la capacité de la SCS de recueillir suffisamment de plasma pour
répondre aux besoins de la population canadienne », précise Adrienne
Silnicki, directrice nationale, politiques et défenses des droits,
Coalition canadienne de la santé. « La ministre fédérale de la Santé
doit donner son appui au système public de collecte du sang, et rejeter
ceux qui essaient de faire des profits avec le sang des personnes du
Canada. »
BloodWatch
demande au premier ministre de s'excuser officiellement auprès des
personnes ayant reçu du sang contaminé, de reconnaître le 26 novembre
comme la journée du dépôt du Rapport Krever à la Chambre des communes,
et d'agir immédiatement pour retirer les permis accordés à toutes les
cliniques privées à but lucratif de collecte de sang.
On estime à 8 000 le nombre de vies perdues au Canada en raison de la tragédie du sang contaminé.
-30-
|
|
Pour en savoir davantage, communiquez avec :
Adrienne Silnicki, directrice nationale, politiques et défense des droits, Coalition canadienne de la santé, 613-402-6793
Lauren Snowball,agente des communications, Fédération canadienne des syndicats d'infirmières et d'infirmiers, 613-868-5702
Kat Lanteigne, directrice générale et co-fondatrice de BloodWatch, 647-272-7381
Paula Doucet, présidente, Syndicat des infirmières et infirmiers du Nouveau-Brunswick, 506-543-2255
*Pour consulter les documents obtenus dans le cadre d'une demande d'accès à l'information, envoyez un courriel à info@bloodwatch.org
|
|
La Coalition
canadienne de la santé est une organisation publique de défense des
droits qui lutte pour préserver et améliorer le régime public
d'assurance-maladie. Pour en savoir davantage sur notre travail : coalitionsanté.ca.
|
Friday, November 17, 2017
Monday, November 13, 2017
Friday, November 10, 2017
Monday, November 6, 2017
The Gluten Free Bar Launches 'Spot the GFB Bear' Contest To Coincide With New Packaging Launch
GRAND RAPIDS, Mich., Nov., 2017 /PRNewswire/ -- The GFB: Gluten Free Bar (GFB),
a leading brand for fun and flavorful gluten-free snacks, is
challenging fans today to go out into the wild and "Spot the GFB Bear"
in its natural habitat at their local grocery and health food store.
Consumers spotting the new GFB bear are encouraged to snap a photo with
the new bear packaging, tag GFB on social media, and use the hashtag
#WildGFBear to enter to win a month's supply of GFB products.
 GFB fans can spot the bear at select locations today through
November 20. The contest coincides with the announcement of GFB's
revamped packaging featuring its new hand drawn bear and colorful
design. The new packaging begins rolling out on GFB Bars across the U.S. this month. The remaining GFB products, including Bites and Power Breakfast, will receive a fresh new look later in 2018.
GFB fans can spot the bear at select locations today through
November 20. The contest coincides with the announcement of GFB's
revamped packaging featuring its new hand drawn bear and colorful
design. The new packaging begins rolling out on GFB Bars across the U.S. this month. The remaining GFB products, including Bites and Power Breakfast, will receive a fresh new look later in 2018.
"The Gluten Free Bear has actually been part of our brand for several years, and incorporating him into more of our packaging brings an element of fun to our products that we felt was missing," said Elliott Rader, co-founder and partner at GFB. "We know that many consumers still equate 'gluten-free' with 'tastes bad.' While there are a lot of great things about our snacks, we pride ourselves on making products that taste amazing and we want consumers to know that. After all, eating something that tastes amazing and is better-for-you, should be a fun part of your day. The Gluten Free Bear represents that fun and flavorful component and we let our certifications and ingredients speak to the quality and nutritional aspect of our products."
The GFB specializes in better-for-you, gluten-free snacks, and the product recipes will remain the same throughout the packaging revamp.
"We are simply updating the first impression of our product to more closely match what we believe in and our culture," said Marshall Rader, co-founder and partner at GFB. "Our consumers will have the same product experience they have come to love just with an enhanced look. We think launching the contest is a fun way to show off our new packaging and to engage GFB fans. We are excited to see where our bear will be spotted – don't forget this is a roll-out so you may have to dig through bear imposters – to spot him!"
Guidelines for the "Spot the GFB Bear" contest include:
The Gluten Free Bar (GFB)Established in 2010, Grand Rapids, Michigan-based Gluten Free Bar (GFB) is a leading brand for fun and flavorful gluten-free snacks in the United States and Europe. The company offers health conscious consumers delicious snack varieties that are non-GMO, Certified Vegan, soy-free, dairy-free and Certified Gluten-Free. The Certified B Corporation is known for its sustainable, earth-friendly manner of production. For more information about GFB, please visit www.theglutenfreebar.com.

"The Gluten Free Bear has actually been part of our brand for several years, and incorporating him into more of our packaging brings an element of fun to our products that we felt was missing," said Elliott Rader, co-founder and partner at GFB. "We know that many consumers still equate 'gluten-free' with 'tastes bad.' While there are a lot of great things about our snacks, we pride ourselves on making products that taste amazing and we want consumers to know that. After all, eating something that tastes amazing and is better-for-you, should be a fun part of your day. The Gluten Free Bear represents that fun and flavorful component and we let our certifications and ingredients speak to the quality and nutritional aspect of our products."
The GFB specializes in better-for-you, gluten-free snacks, and the product recipes will remain the same throughout the packaging revamp.
"We are simply updating the first impression of our product to more closely match what we believe in and our culture," said Marshall Rader, co-founder and partner at GFB. "Our consumers will have the same product experience they have come to love just with an enhanced look. We think launching the contest is a fun way to show off our new packaging and to engage GFB fans. We are excited to see where our bear will be spotted – don't forget this is a roll-out so you may have to dig through bear imposters – to spot him!"
Guidelines for the "Spot the GFB Bear" contest include:
- One entry per social media account per day
- Entries must include the hashtag #WildGFBear
- All entries must tag @theGFB social properties
- All entries must be submitted by November 20, 2017 at 11:59 p.m. EST
The Gluten Free Bar (GFB)Established in 2010, Grand Rapids, Michigan-based Gluten Free Bar (GFB) is a leading brand for fun and flavorful gluten-free snacks in the United States and Europe. The company offers health conscious consumers delicious snack varieties that are non-GMO, Certified Vegan, soy-free, dairy-free and Certified Gluten-Free. The Certified B Corporation is known for its sustainable, earth-friendly manner of production. For more information about GFB, please visit www.theglutenfreebar.com.
SOURCE The Gluten Free Bar (GFB)
CONTACT: Megan McCarl, mmccarl@lambert.com, 616-233-0500
Subscribe to:
Comments (Atom)